-->
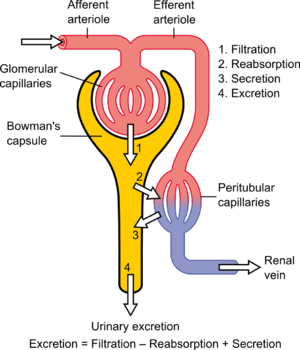
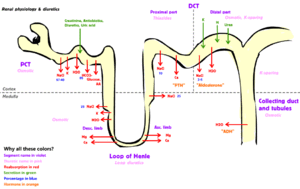
A lymph node is a small ball or an oval-shaped organ of the immune system, distributed widely throughout the body including the armpit and stomach/gut and linked by lymphatic vessels. Lymph nodes are garrisons of B, T, and other immune cells. Lymph nodes are found all through the body, and act as filters or traps for foreign particles. They are important in the proper functioning of the immune system. They are packed tightly with the white blood cells called lymphocytes and macrophages.

Lymph nodes also have clinical significance. They become inflamed or enlarged in various conditions, which may range from trivial, such as a throat infection, to life-threatening such as cancers. In the latter, the condition of lymph nodes is so significant that it is used for cancer staging, which decides the treatment to be employed, and for determining the prognosis.
Lymph nodes can also be diagnosed by biopsy whenever they are inflamed. Certain diseases affect lymph nodes with characteristic consistency and location.
-->
A lymph node is a small ball or an oval-shaped organ of the immune system, distributed widely throughout the body including the armpit and stomach/gut and linked by lymphatic vessels. Lymph nodes are garrisons of B, T, and other immune cells. Lymph nodes are found all through the body, and act as filters or traps for foreign particles. They are important in the proper functioning of the immune system. They are packed tightly with the white blood cells called lymphocytes and macrophages.

Lymph nodes also have clinical significance. They become inflamed or enlarged in various conditions, which may range from trivial, such as a throat infection, to life-threatening such as cancers. In the latter, the condition of lymph nodes is so significant that it is used for cancer staging, which decides the treatment to be employed, and for determining the prognosis.
Lymph nodes can also be diagnosed by biopsy whenever they are inflamed. Certain diseases affect lymph nodes with characteristic consistency and location.



 11:35 AM
11:35 AM
 Unknown
Unknown